44 lewis symbols
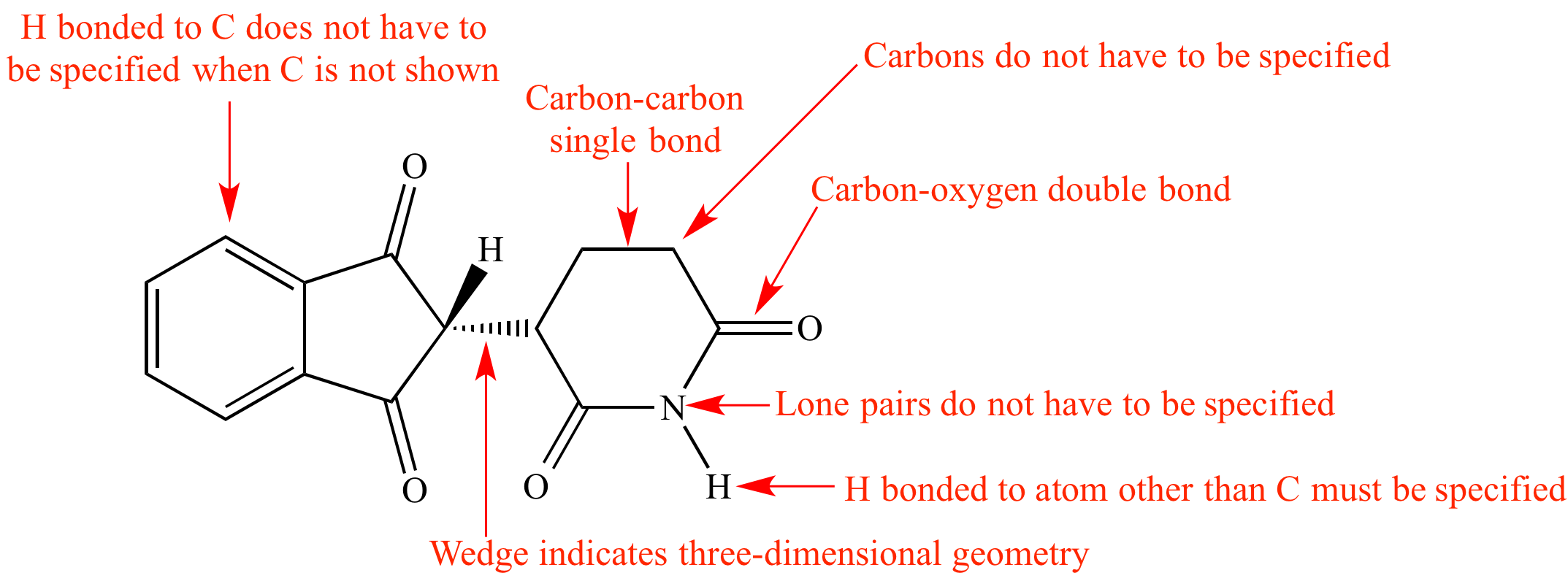
Lewis Dot Symbols and Lewis Structures | Boundless Chemistry | | Course ... Lewis symbols represent the valence electrons as dots surrounding the elemental symbol for the atom. Key Terms group: A column in the periodic table that consists of elements with similar chemical reactivity, because they have the same number of valence electrons. Lewis structure - Wikipedia Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Lewis Symbols and Structures - Chemistry 2e We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: (Figure) shows the Lewis symbols for the elements of the third period of the periodic table.

Lewis symbols
The Lewis Electron-Dot Symbols of Elements A Lewis symbol is a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. Each dot represents one electron. Hydrogen: Oxygen: Chlorine: Chloride ion: How To Draw the Lewis Structure For an Element Lewis Symbols and Structures (4.4) - Chemistry 110 Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.9 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis Symbols Flashcards | Quizlet What are lewis symbols? the dots representing the valance electrons in the atom. how do we write them? 1. Determine # of valence electrons 2. Put that number of dots around elemental symbol. since electrons have a 1- charge, they want to be_____ from each other. far away.
Lewis symbols. Lewis Structure | Encyclopedia.com Lewis symbols are a simple way of visualizing the valence electrons in an atom. In a Lewis symbol, the symbol for the element is used to represent the atom and its core electrons. Dots placed around the atom are used to indicate the valence electrons. When combined to form Lewis structures, Lewis symbols make it possible to predict the shape of ... Lewis Symbols and Structures - Chemistry Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: [link] shows the Lewis symbols for the elements of the third period of the periodic table. Lewis Symbols and Structures - chem-textbook Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: [link] shows the Lewis symbols for the elements of the third period of the periodic table. Lewis Symbols or Diagrams - Elmhurst University A Lewis Symbol consists of the element symbol surrounded by "dots" to represent the number of electrons in the outer energy level as represented by a Bohr Diagram. The number of electrons in the outer energy level is correlated by simply reading the Group number.
Lewis symbols | Valency | Number of bonds each element makes - Dr K Lewis symbols are often referred to as electron-dot symbols as well. We will then use th... In this video, we will go through an easy way to draw Lewis symbols. Lewis Dot Structures - YouTube Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how... 4.4 Lewis Symbols and Structures - General Chemistry 1 & 2 We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. 7.3 Lewis Symbols and Structures - Chemistry We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1.
Lewis Structure Definition and Example - ThoughtCo A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons. Lewis Structures: Dot Symbols, Diagram, Examples - Embibe In a Lewis Structure, electrons are represented as "dots" surrounding the central metal atom. The central metal is denoted by using its chemical symbol from the Periodic Table. Learn Exam Concepts on Embibe In Lewis Structures, a line is used to represent the bonding electrons between two combining atoms. Lewis Symbols and Structures - Introductory Chemistry The following table demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. Lewis Structures Lewis Symbols and Structures | Introductory Chemistry - Lecture & Lab ... We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1.
7.3 Lewis Symbols and Structures - Chemistry We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1.
Lewis Symbols and the Octet Rule | Chemistry | JoVE The Lewis symbol for neon has eight dots, two dots on each side, representing the filled electron configuration; in other words, an octet. The octet rule states that an atom tends to lose, gain, or share electrons in the form of bonds until a stable electron configuration, an octet, is reached. Consider carbon dioxide.
Lewis Symbols - Class Notes G.N. Lewis introduced simple symbols to denote the valence electrons in an atom. The outer shell electrons are shown as dots surrounding the symbol of the atom. These symbols are known as Lewis symbols are electron dot symbols. Significance The number of dots around the symbol give the number of electrons present in the outermost shell.
Chemical Bonding and Lewis Symbols - organicmystery.com Significance of Lewis Symbols. The dots on Lewis symbol help us calculate the valency of the element. The combining power of an element is known as its valency. Valency helps us find out how many electrons an atom needs to gain, lose, or share to form a bond with other atom. For example, sodium has only one electron in its outermost shell ...
7.3 Lewis Symbols and Structures - Chemistry 2e | OpenStax We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.
6.1 Lewis Symbols and Structures - Chemistry Fundamentals Figure 6.1.1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 6.1.1 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:
Lewis Symbols | Practice Questions - organicmystery.com Lewis SymbolsPractice Questions. Lewis Symbols. We discussed in chemical bonding and Lewis symbols that Lewis dot symbols are used to represent valence electrons. Now, it's time you answered some questions on Lewis symbols. Write Lewis symbol of Mg. Write Lewis symbols of S and S 2−. The atomic number of sulphur is 16.
Lewis Symbols | Chemical Bonding - Nigerian Scholars Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: The figure below shows the Lewis symbols for the elements of the third period of the periodic table.
Glass Lewis opposes lawyer election to SoftBank board for second year To add symbols: Type a symbol or company name. When the symbol you want to add appears, add it to My Quotes by selecting it and pressing Enter/Return. Copy and paste multiple symbols separated by ...





Post a Comment for "44 lewis symbols"