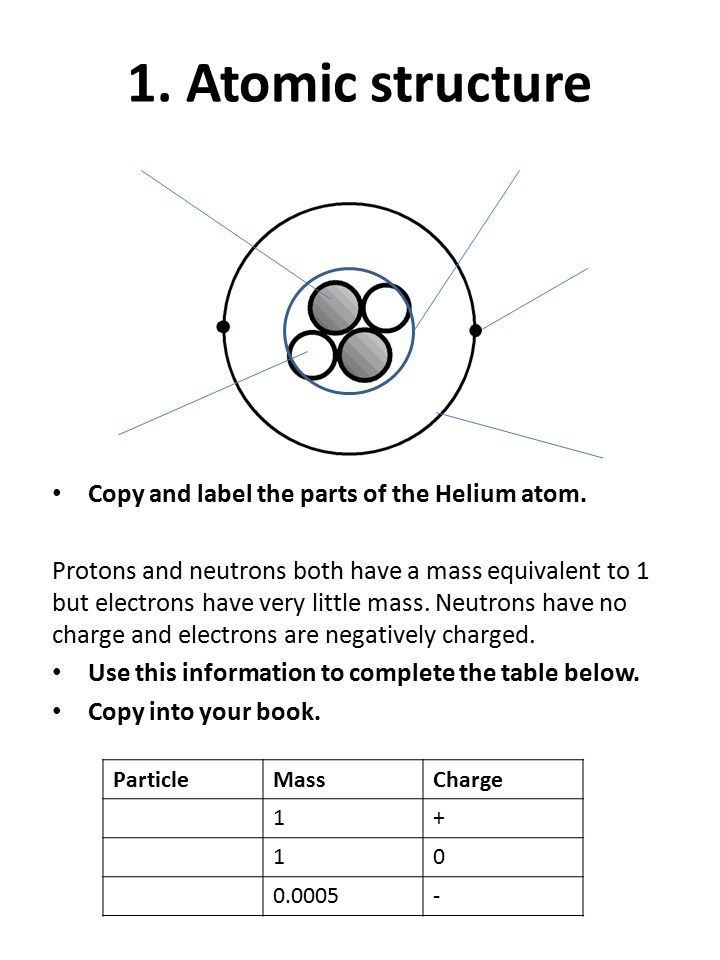
44 parts of a helium atom
The Parts of the Periodic Table - Angelo State University Ionization energies reported in unites of kilojoules per mole (kJ/mol). Data taken from John Emsley, The Elements, 3rd edition.Oxford: Clarendon Press, 1998. The ionization energy of an atom is the amount of energy that is required to remove an electron from a mole of atoms in the gas phase:. M(g) ® M + (g) + e- It is possible to remove more electrons from most elements, so … Octet Rule - Detailed Explanation with Examples, Exceptions - BYJUS An ion, atom, or a molecule containing an unpaired valence electron is called a free radical. These species disobey the octet rule. However, they are very unstable and tend to spontaneously dimerize. Since the first shell can only accommodate two electrons, elements such as lithium, helium, and hydrogen obey the duet rule instead of the octet ...
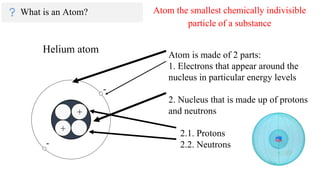
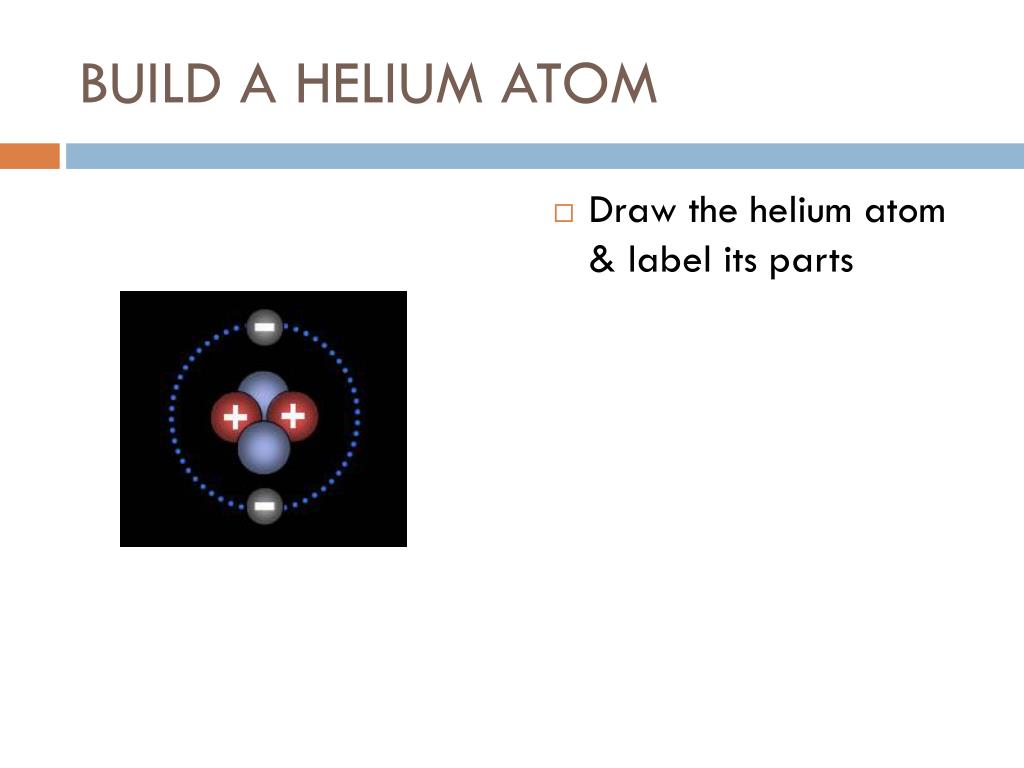
Elements and Compounds Printable Worksheets - TeAch … Parts of An Atom - Label the parts of the atom pictured below. Make sure to include all of the words in the word bank. ... Atom: Elements can consist of just one atom, like Helium (He) Ion: For example, copper ion (Cu ++), Sodium ion (Na +) Isotope: Carbon has 3 isotopes, Carbon 12, Carbon 13, and Carbon 14.

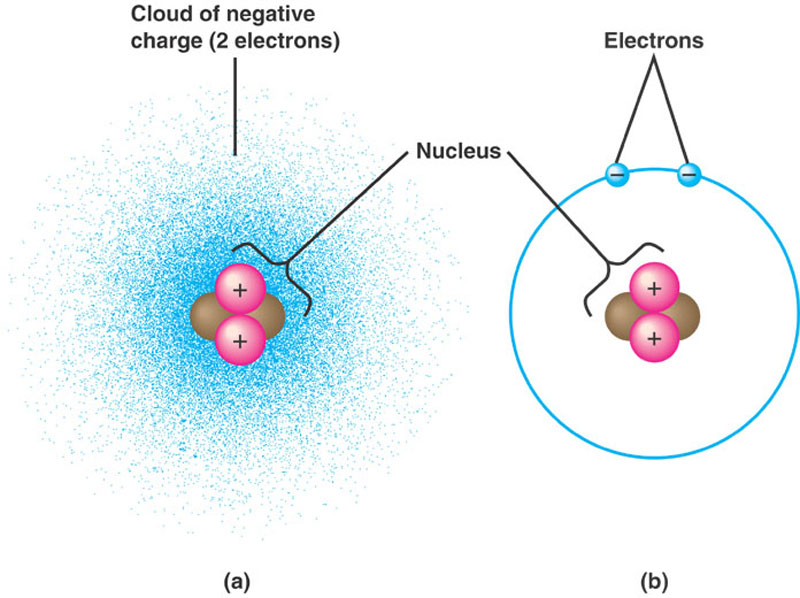
Parts of a helium atom
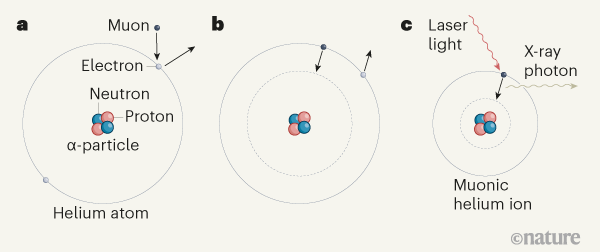
Helium Energy Levels - GSU The helium ground state consists of two identical 1s electrons. The energy required to remove one of them is the highest ionization energy of any atom in the periodic table: 24.6 electron volts. The energy required to remove the second electron is 54.4 eV, as would be expected by modeling it after the hydrogen energy levels . Helium - Element information, properties and uses | Periodic Table Helium can be found in certain parts of the world, notably in Texas, as a minor component in some sources of natural gas. The interesting thing is how this gas gets into the ground in the first place. ... This alpha-particle is actually just the heart of a helium atom - its nucleus. Once it has grabbed a couple of electrons, a helium atom has ... What is Nitrogen?(N) - Chemical Properties, Cycle & Uses with … Nitrogen Uses and Properties - Nitrogen is a diatomic gas with atomic number 7 and symbol N. Know the nitrogen atomic number, the atomic mass of nitrogen and atomic weight of nitrogen. Explore the Uses of Nitrogen.
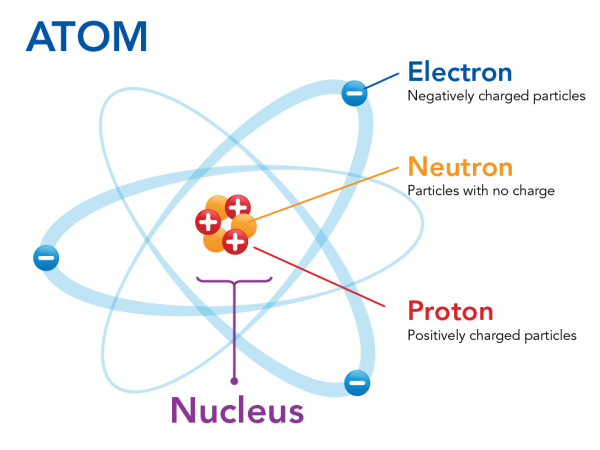
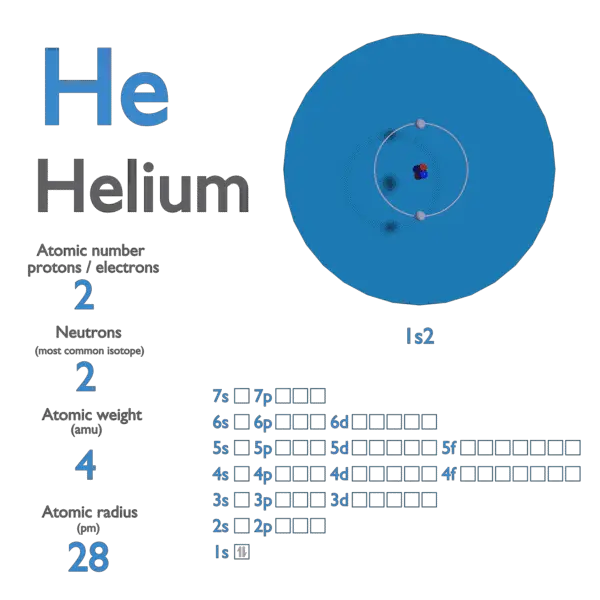
Parts of a helium atom. Atom Quiz - ThoughtCo Mar 08, 2017 · The atomic number of an atom is the same as the number of protons it has. For example, hydrogen has one proton and is atomic number 1. For example, hydrogen has one proton and is atomic number 1. Each helium atom has two protons, so the element is atomic number 2. Number of Protons, Neutrons, and Electrons in an Atom 02/06/2019 · The three parts of an atom are positive-charged protons, negative-charged electrons, and neutral neutrons. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. ... If the atomic weight is 4.001, you can be confident the atom is helium, with 2 protons and 2 neutrons. An atomic weight closer ... Atomic Design Methodology | Atomic Design by Brad Frost Each atom in the natural world has its own unique properties. A hydrogen atom contains one electron, while a helium atom contains two. These intrinsic chemical properties have profound effects on their application (for example, the Hindenburg explosion was so catastrophic because the airship was filled with extremely flammable hydrogen gas ... Loading... Loading... ... Loading...
Helium-4 - Wikipedia Helium-4 (4 He) is a stable isotope of the element helium.It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons.. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 … Kids science: Periodic Table of Elements - Ducksters Each horizontal row in the table is a period. There are seven (or eight) total periods. The first one is short and only has two elements, hydrogen and helium. The sixth period has 32 elements. In each period the left most element has 1 electron in its outer shell and the right most element has a full shell. Groups What is Nitrogen?(N) - Chemical Properties, Cycle & Uses with … Nitrogen Uses and Properties - Nitrogen is a diatomic gas with atomic number 7 and symbol N. Know the nitrogen atomic number, the atomic mass of nitrogen and atomic weight of nitrogen. Explore the Uses of Nitrogen. Helium - Element information, properties and uses | Periodic Table Helium can be found in certain parts of the world, notably in Texas, as a minor component in some sources of natural gas. The interesting thing is how this gas gets into the ground in the first place. ... This alpha-particle is actually just the heart of a helium atom - its nucleus. Once it has grabbed a couple of electrons, a helium atom has ...
Helium Energy Levels - GSU The helium ground state consists of two identical 1s electrons. The energy required to remove one of them is the highest ionization energy of any atom in the periodic table: 24.6 electron volts. The energy required to remove the second electron is 54.4 eV, as would be expected by modeling it after the hydrogen energy levels .


























Post a Comment for "44 parts of a helium atom"