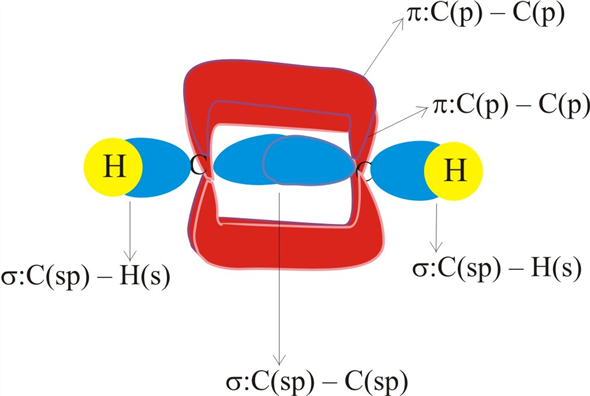
40 label all bonds on the sketch of the structure.
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B(p) - F(p) Empty p orbital Lone pair in p orbital B B(sp²) - F(p) в : В(8) — F(p) o : B(p) - F(p) Empty sp? orbital Solved In the sketch of the structure of NF3 label all | Chegg.com Transcribed image text: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
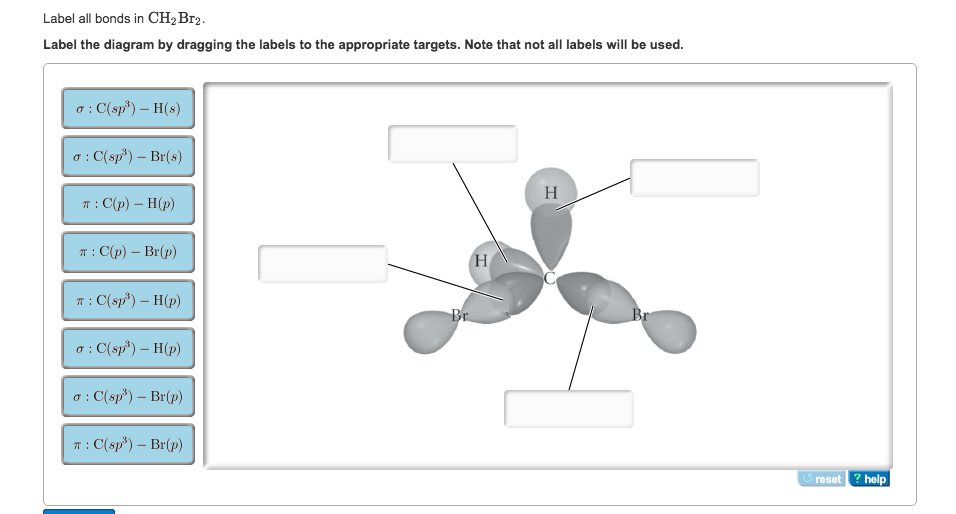
OneClass: Label all bonds in CH2Br2? Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 634. views. For unlimited access to Homework Help, a Homework+ subscription is required. ... Chemistry: Structure and Properties. 2nd Edition, Tro. ISBN: 9780134293936. Related questions. Which compound contains both ionic and covalent bonds? A. KI. B. CaCl 2. C. CH 2 Br 2.

Label all bonds on the sketch of the structure.
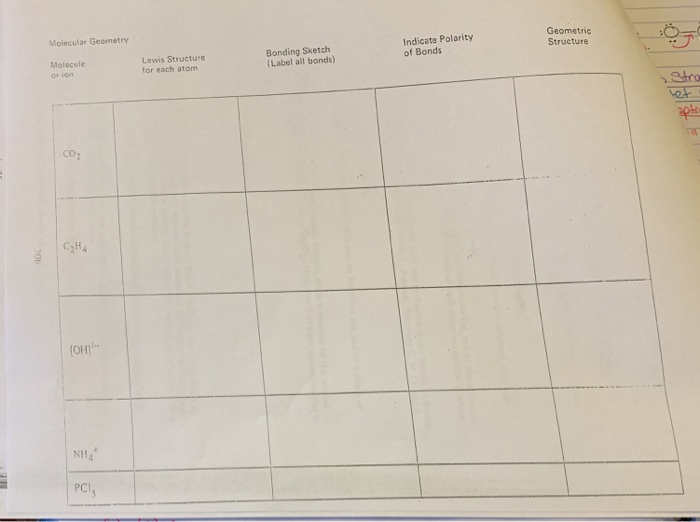
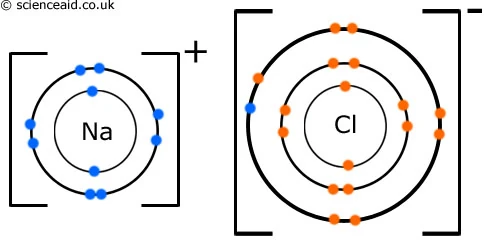
Ch 2 Chemistry Flashcards | Quizlet Ionic bonds involve electrons being donated from one atom to another. How are the electrons treated in covalent bonds? ... Label a portion of the molecule below where the primary structure is visible; label two types of secondary structure; label the tertiary structure. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b. C2H4 (skeletal structure H2CCH2) c. C2H6 (skeletal structure H3CCH3) Answer. a) Both the C atoms have two electron groups. So the electron geometry is linear and the SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. SO3 2- b. PF6- c. BrF3 d. HCN Answer a) The S atom has four electron groups. So the electron geometry is tetrahedral and the hybridization is s p 3 . b) The P atom has six electron groups with no lone pairs.
Label all bonds on the sketch of the structure.. Solved In the sketch of the structure of BF3 label all | Chegg.com Expert Answer 100% (32 ratings) Transcribed image text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape NH3 Bond angles. There are three single bonds and one lone pair of electrons in NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3 Answer a) The interior C atom has three electron groups. So the electron geometry is trigonal planar and the hybridization is s p 2 . PDF SECTION ATOMS, IONS, AND MOLECULES 2.1 Study Guide Sketch the structure of an atom. Label the protons, neutrons, nucleus, and electrons. 3. ... All atoms have the same basic structure, composed of three smaller particles. ... different elements can link, or bond, together to form compounds. Atoms form bonds
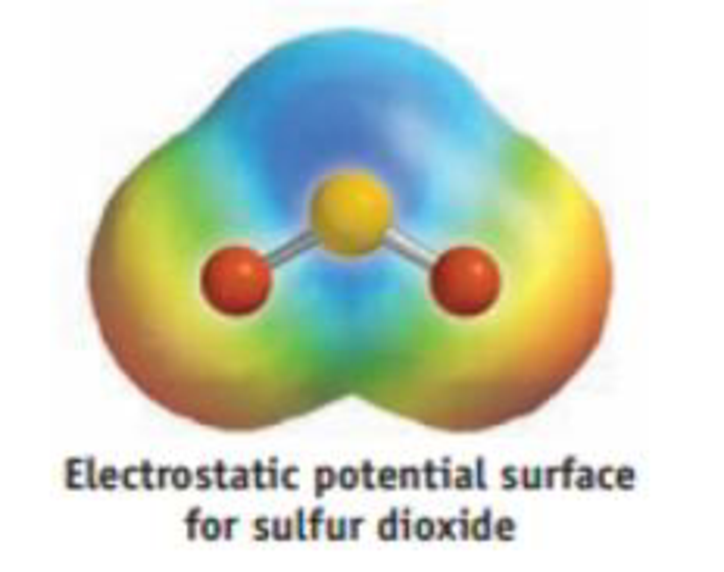
Answered: In the sketch of the structure of… | bartleby Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. CHEM: Chapter 10 Flashcards - Quizlet Determine the geometry about each interior atom in each molecule and sketch the molecule. (Skeletal structure is indicated in parentheses.) a. CH3NH2 (H3CNH2) b. CH3CO2CH3 (H3CCOOCH3 One O atom attached to 2nd C atom; the other O atom is bonded to the 2nd and 3rd C atom) c. NH2CO2H (H2NCOOH both O atoms attached to C) Molecular Shape and Polarity Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom. Answered: In the sketch of the structure of SO2… | bartleby Transcribed Image Text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - 0 (sp²) т: S (p) — О (p) T: S (sp²) - O (p) r: S (sp²) - O (p ...
Answered: In the sketch of the structure of NF3… | bartleby Chemistry Q&A Library In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. σ: Νip) - F(p) Lone pair in sp orbital 1L σ: Nip) - F (sp') T:N(sp³) - F(p) Lone pair in p orbital T: N(p) - F(p) Lone pair in s orbital σ: Ν(sp ... Label All Bonds In Bf3 - Structure And Bonding Of New Boron And Carbon ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in examples 6.1 and 6.2. In the sketch of the structure of bf3 label all bonds. Four electron pairs are distributed in a tetrahedral shape. Labels can be used once, . Draw hybridized orbitals around central atom 4. In the sketch of the structure of ... PDF Tro Chemistry - A Molecular Approach 2nd Edition Sketch the structure, eluding overlapping orbitals, and label all bonds using notation shown in Examples 10.6 and 10.7. a. N2H2 (skeletal structure HNNH) b. N2H4 (skeletal structure H2NNH2) c. CH3NH2 (skeletal structure H3CNH,) 66. Write a hybridization and bonding scheme for each molec that contains more than one interior atom. SOLVED:Write a hybridization and bonding scheme for each molecule ... VIDEO ANSWER: All right, So for this one, we're gonna need to look at higher ization of orbital's and a little bit of molecular geometry. ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CCl4 b. NH3 c. OF2 d. CO2. Answer (a) See solution (b) See solution (c) See ...
Solved Label all bonds on the sketch of the structure, Drag | Chegg.com Label all bonds on the sketch of the structure, Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help Nspr") - H () .: N (p) - H (a) Nap) - NPP) H Н N (P)-NCp) Lone parin N (sp) Lone pair in N (p) : N (P) - N (P) Н H Nap')-Nap Lone parin N (7) Question: Label all bonds on ...
Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7 a. COCl2 (carbon is central atom b. BrF5 c. XeF2 d. I3- ... 65: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom.
Post Lecture Lewis Structures, Problem Set #6 (Ch 5), Problem ... - Quizlet Draw an appropriate Lewis structure for IF5. Identify the geometry of IF5 using VSEPR theory. Specify whether the molecule IF5 is polar or nonpolar and explain why. Identify the hybridization of all interior atoms for the molecule IF5, according to valence bond theory, in the diagram showing orbital overlap below.
Answered: Write a hybridization and bonding… | bartleby Sketch the structure, including overlapping orbitals, andlabel all bonds using the notation shown in Examples 6.1 and 6.2.a. COCl2 (carbon is the central atom)b. BrF5c. XeF2d. I3 Question Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and
b. $\mathrm{N}_{2} \mathrm{H}_{4}\left(\right.$ skeletal structure ... Following this we have C H three n h two 14 Valence Electrons have a carbon interacting in a segment bond with a nitrogen. Nitrogen has a lone pair. ... Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples $10.6$ and $10.7$. ... and label all bonds using the notation shown in Examples ...
Chapter 6, Chemical Bonding II Video Solutions, Chemistry - Numerade Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b.
Solved 1. Label all bonds on the sketch of the structure | Chegg.com 1. Label all bonds on the sketch of the structure (CH3NH2) Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Show transcribed image text Expert Answer Transcribed image text: Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Problem 34 Hard Difficulty Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 Answer (a) See solution (b) See solution (c) See solution (d) See solution View Answer Discussion
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. SO3 2- b. PF6- c. BrF3 d. HCN Answer a) The S atom has four electron groups. So the electron geometry is tetrahedral and the hybridization is s p 3 . b) The P atom has six electron groups with no lone pairs.


Post a Comment for "40 label all bonds on the sketch of the structure."